India approves survivin drug to treat blood cancer: All about Curtemi and how it works
The second CAR-T cell therapy, Curtem, has been approved by India’s drug regulator. Curtemi is a “survival medicine” for blood cancer patients.
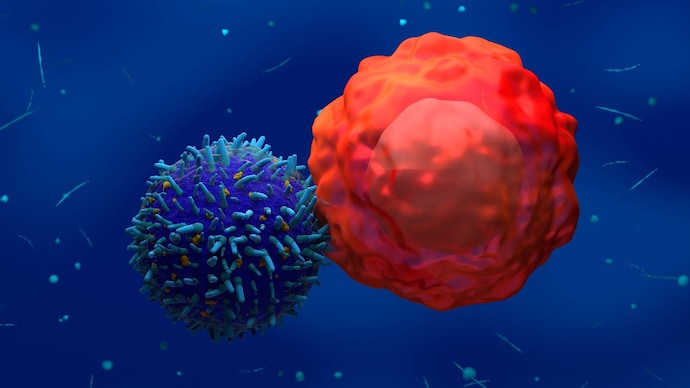
A “survival drug” has been approved in India for blood cancer patients suffering from advanced or relapsed stages of the disease. Bengaluru-based biotech startup, Immunil Therapeutics, has launched Curtem, a CAR-T cell therapy for patients with B-cell non-Hodgkin lymphoma (B-NHL).
The drug is the second CAR-T cell therapy approved in India after the Central Drugs Standard Control Organization (CDSCO) approved homegrown NexCAR19 developed by ImmunoAct, a company based at the Indian Institute of Technology Bombay (IITB). Tata Memorial Hospital.
A “living drug” differs from a traditional chemical drug in that it is made from cells to elicit a long-lasting immune response. These drugs are cell therapies that are removed from the patient, modified and then put back into the patient. This is called CAR-T cell therapy which is a type of immunotherapy that genetically engineers the patient’s T cells to attack cancer cells.
Qartemi, a personalized chimeric antigen receptor, or CAR-T cell, is now available locally for adult patients with relapsed or refractory B-NHL.
India is facing a rising burden of blood cancers, with about 1,20,000 new cases reported every year and more than 70,000 deaths from leukemia, lymphoma and multiple myeloma. With this, Qartemi provides hope to patients for whom conventional treatments, including chemotherapy, prove ineffective.
The company developing the drug is a gene and cell therapy startup backed by Biocon founder Kiran Mazumdar Shaw and oncologist and author Dr Siddharth Mukherjee.
“By offering Curtemi at a significantly accessible price compared to global alternatives, we are able to indigenously develop this therapy at our facility in Bangalore to ensure global standards of safety and efficacy,” Amit Muqim, CEO of Immunil Therapeutics, said in a statement. Take pride in developing.”
The therapy costs between Rs 35 lakh and Rs 50 lakh per treatment, which the company says is significantly more affordable than similar global options.
In 2022, the company launched a CAR T-cell therapy trial focused on Qartemi, designed for patients with recurrent or refractory B-cell malignancies. Since such patients received limited treatment during relapse or when refractory (not responding to drugs).
The trials, called IMAGINE, were conducted at three major medical institutions: Narayana Hospital in Bengaluru, Apollo Cancer Hospital in Chennai and PGIMER in Chandigarh.
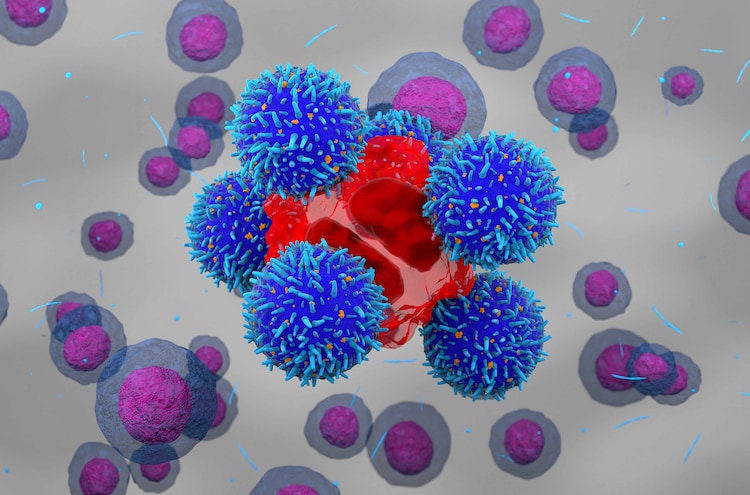
Results of the IMAGINE trial showed that Qartemi was safer and more effective than other CAR-T cell therapies approved by the US Food and Drug Administration (FDA). The Phase 2 clinical trials were supported by BIRAC, an agency of the Department of Biotechnology, through its Biotechnology Industry Partnership Program (BIPP) scheme.
In Phase 2 trials, the drug achieved an 83.3% overall response rate.
The company has partnered with several hospitals, including Narayana Health, Apollo Hospitals, CMC Vellore and Ludhiana, Manipal Hospital, RGCIRC Delhi, SGPGI Lucknow, Amrita Hospital Faridabad, HOC Vedanta Ahmedabad, Sitecare Bangalore, Sparsh Bangalore, Marengo Asia Hospital . Qartemi to his patients.
Qartemi, known as “India’s first international CAR T-cell therapy”, has received the license from Hospital Clínic de Barcelona (HCB), a globally renowned institute leading in cell therapy innovation.