Drug regulator suspends approval of eye drops claiming to replace reading glasses
India’s drug regulator has suspended the permission granted to manufacture and sell its new eye drops, Presview, which claimed to remove reading glasses.
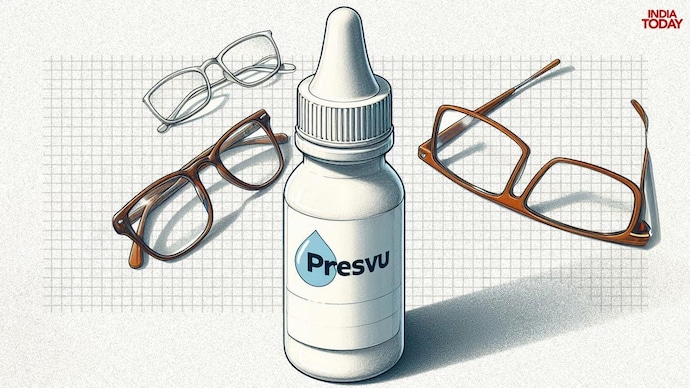
Taking seriously Mumbai-based Entode Pharmaceuticals’ claim that it can help reduce dependence on reading glasses for people suffering from presbyopia, the Drugs Controller General of India has suspended the permission granted for the manufacture and sale of its new eye drops.
According to the National Eye Institute, presbyopia is a refractive error that makes it difficult for middle-aged and older people to see things up close.
The drug regulator said the company made claims for a drug product for which it had not obtained approval from the Central Licensing Authority, thereby violating the provisions of the New Drugs and Clinical Trials Rules, 2019.
In an order issued on September 10, the Drugs Controller General of India (DCGI) said the directorate had issued permission on August 20 for the manufacture and marketing of Pilocarpine Hydrochloride Ophthalmic Solution for the treatment of presbyopia in adults.
Subsequently, on September 4, the drug regulator had sought clarification from the company for the claims made in the press, following which the drug manufacturer had also submitted its reply.
In response to the claim of “the first eye drop made in India to reduce the need for reading glasses”, the order said the company stated that currently there are no other eye drops approved for the treatment of presbyopia in India.
“In this regard, you are hereby informed that Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25% w/v has not been approved for any claim that it is designed to reduce the need for reading glasses,” the order said.
In response to the claim that “this eye drop provides a non-invasive alternative that can enhance near vision without the need for reading glasses”, the company submitted that in the clinical trials conducted, subjects did not wear glasses to participate.
The order said, “In this regard, you are informed that Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25 PC w/v is approved for the treatment of presbyopia in adults and is not approved for the claim that these eye drops can enhance near vision without the need for reading glasses.”
In response to the claim that “PressVu can provide an advanced alternative that enhances near vision within 15 minutes”, the firm said in its reply that a doctor had evaluated the pharmaceutical product in comparison to reading glasses.
“In this regard, you are informed that Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25 PC w/v has been approved for the treatment of presbyopia in adults and is not approved for the claim that PresVue may provide an improved alternative that improves near vision within 15 minutes,” the order said.
The drug regulator said the company failed to answer questions and attempted to justify claims for a product for which no approval had been granted.
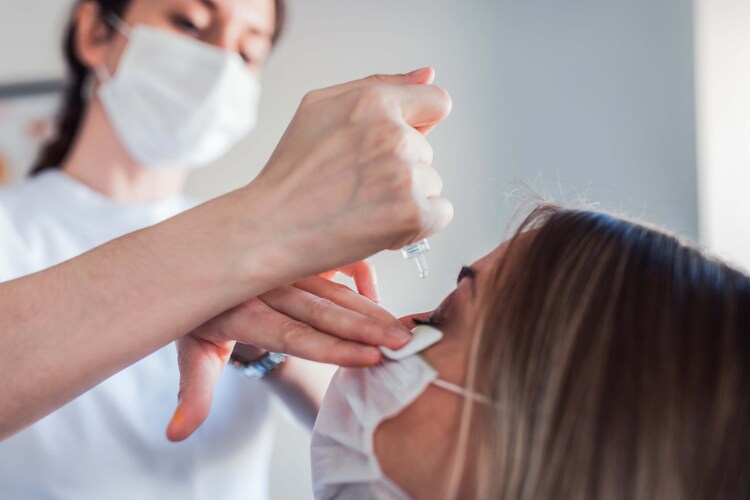
The order said, “It is evident that you have not obtained any prior approval from the Central Licensing Authority to make such claims for the above-mentioned drug product. Thus, you have violated condition no. (vi) of the Permission dated 20.08.2024 issued to you for manufacturing and marketing of Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25% w/v under the provisions of the New Drugs and Clinical Trials Rules, 2019.”
The order further said that considering various media reports, there is a likelihood that the general public may be mislead by the claims made by the company, for which no approval was granted.
The order said, “In view of the above and considering public interest, the permission issued for manufacture and marketing of Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25% w/v under the provisions of Rule 84 of the Drugs and Cosmetics Act, 1940 read with New Drugs and Clinical Trial Rules, 2019, is suspended till further orders.”



